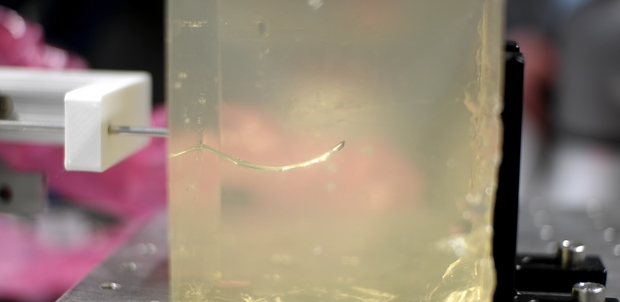Signal and Image Processing
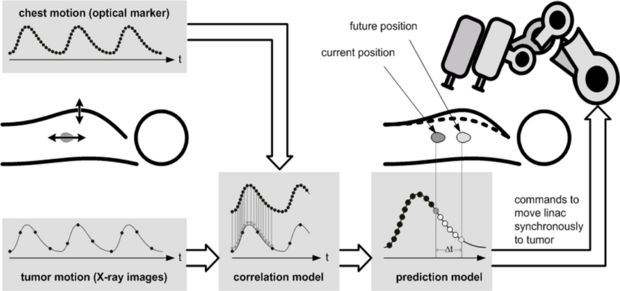
Motion is an important aspect in medical interventions. Predicting patient motion can improve the quality of treatment for example in radiosurgery. We develop systems and different algorithms to detect and compensate for motion. In some scenarios, additional differentiation between healthy and abnormal tissue structures is beneficial. The detection of malignant tissue structures can support in different navigation tasks. We explore different imaging modalities for shear wave elastography.
Selected Publications
- M. Neidhardt, R. Mieling, M. Bengs, A. Schlaefer (2023). Optical force estimation for interactions between tool and soft tissues. Scientific Reports - Nature. 13. (1), 506 [Abstract]
[doi][www][BibTex]
- S. Latus, S. Grube, T. Eixmann, M. Neidhardt, S. Gerlach, R. Mieling, G. Hüttmann, M. Lutz, A. Schlaefer (2023). A Miniature Dual-Fiber Probe for Quantitative Optical Coherence Elastography. IEEE Transactions on Biomedical Engineering. 70. (11), 3064-3072 [Abstract]
[doi][BibTex]
- J. Sprenger, M. Bengs, S. Gerlach, M. Neidhardt, A. Schlaefer (2022). Systematic analysis of volumetric ultrasound parameters for markerless 4D motion tracking. International Journal of Computer Assisted Radiology, Surgery. [Abstract]
[doi][www][BibTex]
- M. Neidhardt, M. Bengs, S. Latus, S. Gerlach, C. J. Cyron, J. Sprenger, A. Schlaefer (2022). Ultrasound Shear Wave Elasticity Imaging with Spatio-Temporal Deep Learning. IEEE Transactions on Biomedical Engineering. 1-1 [Abstract]
[doi][www][BibTex]
-
M. Schlüter, L. Glandorf, J. Sprenger, M. Gromniak, M. Neidhardt, T. Saathoff, A. Schlaefer
(2020).
High-Speed Markerless Tissue Motion Tracking Using Volumetric Optical Coherence Tomography Images.
IEEE International Symposium on Biomedical Imaging
1979-1982.
[Abstract][doi][BibTex]
-
M. Schlüter, L. Glandorf, M. Gromniak, T. Saathoff, A. Schlaefer
(2020).
Concept for Markerless 6D Tracking Employing Volumetric Optical Coherence Tomography.
Sensors.
20
(9),
2678.
[Abstract][doi][BibTex]
-
M. Bengs and N. Gessert and M. Schlüter and A. Schlaefer
(2020).
Spatio-Temporal Deep Learning Methods for Motion Estimation Using 4D OCT Image Data.
International Journal of Computer Assisted Radiology and Surgery.
15
(6),
943-952.
[Abstract][doi][www][BibTex]
-
N. Gessert, M. Schlüter, A. Schlaefer
(2018).
A Deep Learning Approach for Pose Estimation from Volumetric OCT Data.
Medical Image Analysis.
46
162-179.
[Abstract][doi][www][BibTex]
-
M. Neidhardt, M. Bengs, S. Latus, M. Schlüter, T. Saathoff, A. Schlaefer
(2020).
4D Deep learning for real-time volumetric optical coherence elastography.
International Journal of Computer Assisted Radiology and Surgery 2020
1861-6429.
[Abstract][doi][www][BibTex]
Motion Tracking
Image guidance and navigation are important in medical treatments. We focus on fast imaging modalities that allow real-time motion estimation and interaction. For example, we consider optical coherence tomography, ultrasound or camera systems for motion tracking.
Markerless Tracking with Optical Coherence Tomography
Optical Coherence Tomography (OCT) provides images with high spatial and temporal resolution. In addition to the image information from the sample surface, we also obtain sub-surface information from the structures. In comparison to conventional tracking cameras this can be beneficial in cases where the structures have smooth surfaces with few distinct features for tracking. In our research, we develop a system for markerless tracking of tissue structures in different scenarios to enable high-speed volumetric motion estimation. We study deep learning methods for precise motion estimation and optimize a setup for actively compensating motion.
Ultrasound for Motion Estimation
We consider 4D-ultrasound for motion estimation in real-time. In contrast to optical coherence tomography, we obtain information from deeper tissue structures. Application scenarios include radiosurgery of liver or prostate, where precise motion estimation is of importance. Particularly, we consider markerless estimation of patient motion based on the tissue structure in the ultrasound images. We systemaically analyze different approaches and evaluate the imaging and system setup as well as deep learning methods for motion estimation.
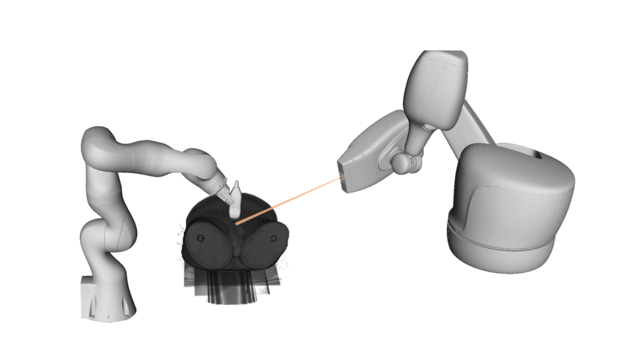
We also study needle steering in medical interventions. The needle detection and tracking can be performed under ultrasound guidance, using either 2D, 3D or 4D ultrasound imaging. We develop experimental setups for reliable needle detection and apply trajectory planning algorithms to reach specific targets.
Shear Wave Elastography
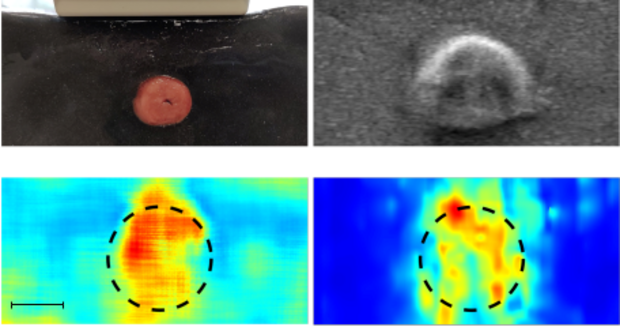
Diseases can lead to a change in mechanical tissue properties. Hence, estimating tissue stiffness helps physicians, for example, in navigation by visualizing tumor regions. We consider Ultrasound and Optical Coherence Tomography as imaging modalities to track the propagation of shear waves in the tissue. In addition to conventional methods for shear wave velocity estimation, we also proposed several deep learning methods to estimate the shear wave velocity more reliably.
Ultrasound Shear Wave Elastography (US-SWE): Ultrasound is a common imaging modality for tracking shear waves and is already implemented in clinical practice. We investigate high-speed US-SWE with linear probes as well as volumetric probes to investigate tissue elasticity and to detect regions with abnormal stiffness values. In our research we are especially interested in machine learning methods for efficient image analysis.
Optical Coherence Elastography (OCE): Optical coherence tomography serves as imaging modality for motion tracking with high spatial and temporal resolution. We characterize tissue structures by evaluating the motion patterns of induced shear waves involving subsurface information. We establish spatio-temporal deep learning methods to estimate tissue stiffnesses from 4D OCT image data. Additionally, we are investigating methods to quantify elasiticities from within the tissue with novel OCE probes. Application scenarios are intravascular shear waves tracking, e.g., in coronary arteries, or quantifying the tissue mechanics by evaluating deformations in front of a OCE needle.
Contact
- Alexander Schlaefer (schlaefer(at)tuhh.de)
- Sarah Latus (s.latus(at)tuhh.de)
- Maximilian Neidhardt (maximilan.neidhardt@tuhh.de)
- Sarah Grube (sarah.grube(at)tuhh.de)